 Iodine and hydrogen react non-spontaneously to produce hydrogen iodide: All the hydrogen halides are soluble in water, in which they form strong acids (with the exception of \(HF\)). Produces iron(III) bromide. fluorine Peroxides, of the form MO2, are formed for all these elements except beryllium as shown: \[M(s) + O_2(g) \rightarrow MO_2(s) \label{13}\].
Iodine and hydrogen react non-spontaneously to produce hydrogen iodide: All the hydrogen halides are soluble in water, in which they form strong acids (with the exception of \(HF\)). Produces iron(III) bromide. fluorine Peroxides, of the form MO2, are formed for all these elements except beryllium as shown: \[M(s) + O_2(g) \rightarrow MO_2(s) \label{13}\]. BEach fluorine atom gains two or more electrons. Reactions are shown below. WebA 15 g sample of lithium is reacted with 15 g of fluorine to form lithium fluoride. WebThe complete transfer of an electron from lithium to fluorine results in a stable compound in which both atoms have full outermost shells. From a standard reduction potential table, it is determined that iodine and bromine cannot oxidize water to oxygen because they have smaller reduction potentials than oxygen. These solutions are good oxidizing agents. Their extended octets allow them to bond with many oxygen atoms at a time.
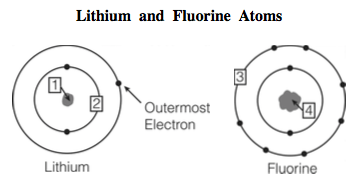
 Lithium, the first metal in Group 1, reacts with Web6 Lithium and fluorine react to form lithium fluoride. All of Group 1 elementslithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: \[H_2 + O_2 \rightarrow H_2O_2 \label{2}\]. 2Li (s) + F2 (g) ----> 2LiF (s) After the reaction is complete, what will be present? At room temperature fluorine is a yellow gas, chlorine is a pale green gas, bromine is a red liquid, and iodine is a purple solid. In fact, lithium and fluorine will react together as shown in the figure below. Astatine is a radioactive element, and exists in nature only in small amounts. 6. The bond strength of these molecules decreases down the group: \(HF > HCl > HBr > HI\). It has 9 electrons that All the oxides and hydroxides of the group 2 metals, except of those of beryllium, are bases: \[ M(OH)_2(s) \rightarrow M^{2+}(aq) + 2OH^-(aq) \label{15}\]. However, current fluoride-ion batteries have poor cyclability that is, they tend to degrade rapidly with charge-discharge cycles. They have high ionization energies and form the most electronegative group of elements. The three metals in this group have many different oxide compounds due to their, Reactions of Selenium and Oxygen. Lithium fluoride and hydrogen fluoride, also known as **20points** When this chemical reaction occurs, the product is A) a salt called lithium fluoride. The other alkali metals (Rb, Cs, Fr) form superoxide compounds (in which oxygen takes the form O2-) as the principal combustion products. These elements vary in their reactions with oxygen. D) the decomposition of two elements, lithium and fluorine. What are the names of the third leaders called? All the halogens exist as diatomic molecules. Their research was printed Dec. 7 in the Journal of Materials Chemistry. Elements such as fluorine, chlorine, bromine, iodine, and astatine belong to Group 17, the halogen group. Their electron configuration, ns, Reactions of Main Group Elements with Carbonates, Reactions of Main Group Elements with Hydrogen, status page at https://status.libretexts.org, \(M\) represents any metal from Group 2 and. Astatine is a radioactive element, and exists in nature only in small amounts. While lithium foils without the CoF 2 @C-modified separator (as shown in Figure 12e,f) show uneven lithium deposition with many patches and pores on the surface, this Thallium is the only element in this group favors the formation of oxide over trioxide. WebCrystal structures and superconductivity of lithium and fluorine implanted gold hydrides under high pressures Gold hydrides in the solid state, as a member of the Au compound family, are rare since the reaction of Au with H is hindered in terms of their similar electronegativity. The other halogens form oxoacids instead of oxides. DEach fluorine atom loses two or more electrons. Because of oxygen's high reactivity, it is most often found in compounds. A molecule with molecular weight of 180.18 g/mol is analyzed and found to contain 40.00% carbon, Do I need to know the number of moles of each element to determine the formula of the compound. However, if there is excess oxygen present, it is possible that a small amount of the compound Li2O2 can be formed.
Lithium, the first metal in Group 1, reacts with Web6 Lithium and fluorine react to form lithium fluoride. All of Group 1 elementslithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. The following equation shows the reaction of hydrogen and oxygen to form hydrogen peroxide: \[H_2 + O_2 \rightarrow H_2O_2 \label{2}\]. 2Li (s) + F2 (g) ----> 2LiF (s) After the reaction is complete, what will be present? At room temperature fluorine is a yellow gas, chlorine is a pale green gas, bromine is a red liquid, and iodine is a purple solid. In fact, lithium and fluorine will react together as shown in the figure below. Astatine is a radioactive element, and exists in nature only in small amounts. 6. The bond strength of these molecules decreases down the group: \(HF > HCl > HBr > HI\). It has 9 electrons that All the oxides and hydroxides of the group 2 metals, except of those of beryllium, are bases: \[ M(OH)_2(s) \rightarrow M^{2+}(aq) + 2OH^-(aq) \label{15}\]. However, current fluoride-ion batteries have poor cyclability that is, they tend to degrade rapidly with charge-discharge cycles. They have high ionization energies and form the most electronegative group of elements. The three metals in this group have many different oxide compounds due to their, Reactions of Selenium and Oxygen. Lithium fluoride and hydrogen fluoride, also known as **20points** When this chemical reaction occurs, the product is A) a salt called lithium fluoride. The other alkali metals (Rb, Cs, Fr) form superoxide compounds (in which oxygen takes the form O2-) as the principal combustion products. These elements vary in their reactions with oxygen. D) the decomposition of two elements, lithium and fluorine. What are the names of the third leaders called? All the halogens exist as diatomic molecules. Their research was printed Dec. 7 in the Journal of Materials Chemistry. Elements such as fluorine, chlorine, bromine, iodine, and astatine belong to Group 17, the halogen group. Their electron configuration, ns, Reactions of Main Group Elements with Carbonates, Reactions of Main Group Elements with Hydrogen, status page at https://status.libretexts.org, \(M\) represents any metal from Group 2 and. Astatine is a radioactive element, and exists in nature only in small amounts. While lithium foils without the CoF 2 @C-modified separator (as shown in Figure 12e,f) show uneven lithium deposition with many patches and pores on the surface, this Thallium is the only element in this group favors the formation of oxide over trioxide. WebCrystal structures and superconductivity of lithium and fluorine implanted gold hydrides under high pressures Gold hydrides in the solid state, as a member of the Au compound family, are rare since the reaction of Au with H is hindered in terms of their similar electronegativity. The other halogens form oxoacids instead of oxides. DEach fluorine atom loses two or more electrons. Because of oxygen's high reactivity, it is most often found in compounds. A molecule with molecular weight of 180.18 g/mol is analyzed and found to contain 40.00% carbon, Do I need to know the number of moles of each element to determine the formula of the compound. However, if there is excess oxygen present, it is possible that a small amount of the compound Li2O2 can be formed.  It can be produced directly from limestone, or as a by-product by Solvay Process.
It can be produced directly from limestone, or as a by-product by Solvay Process.  A 15 g sample of lithium is reacted with 15 g of fluorine to E,)zF8t2kK}z?%kB[ 1 Lithium atoms lose an electron when they react. This section describes the chemistry of halogens with the main group elements such as the alkali metals, alkaline earth metals, and Groups 13 and 14. Sulfur reacts directly with all the halogens except iodine. is. Iodine pentoxide forms iodic anhydride when reacted with water, as shown: Compounds that are made up of both oxygen and hydrogen are considered to be oxygen acids, or oxoacids. Mishras lab is looking to collaborate with researchers who can synthesize the promising electrides identified in this study and test them in prototype batteries. See answers Advertisement kayla213600 B) a new element is formed Chemistry The Mole Concept Determining Formula 1 Answer anor277 Jan 31, 2017 What else but LiF? >7p;|^"*axY|"p(O +Sl"UU**tYz8. SO2 is mainly used to make SO3, which reacts with water to produce sulfuric acid (recall that nonmetals form acidic oxides). The WebFluorine is the chemical element with the symbol F and atomic number 9. Fluorine and lithium react together to form lithium compound that contains F and Li+ ions. Oxygen is ubiquitous; it comprises approximately 46% of the crust, 21% of the atmosphere, and 61% of the human body. Which candy shares its name with a south American mountain range? All the halogens exist as diatomic molecules. A 15 g sample of lithium is reacted with 15 g of fluorine to When we add in fluoride to conventional electrodes, they swell and shrink dramatically as it charges and discharges, and that can lead to cracking and loss of electrical contact.. Aluminum halides adopt a dimeric structure. Cesium, on the other hand, has a significantly lower activation energy, and so although it does not release as much heat overall, it does so extremely quickly, causing an explosion. In iodine, however, the p orbitals are more diffuse, which means the bond becomes weaker than in chlorine or bromine. It is easily reduced, and therefore act as an effective oxidizing agent. Chlorine monoxide, the anhydride of hypochlorous acid, reacts vigorously with water as shown below, giving off chlorine and oxygen as products. Madsen, Dorte. This energy will be recovered (and overcompensated) later, but must be initially supplied. \[ 4M(s) + 3O_2(g) \rightarrow 2M_2O_3(s) \label{22}\]. Common salt, $$NaCl$$, is Print. Web1. The computerized tests introduced fluoride into the interstitial spaces of the layered electrides dicalcium nitride and yttrium hypocarbide. A student writes three statements about the reaction. form lithium fluoride. needs to gain one more valence electron to make its last energy How can I determine the empirical formula of a compound? z1,0IE2 \[2 M(s) + O_2(g) \rightarrow 2 MO(s) \label{12}\]. In principle, you can add a lot of fluoride ions to conventional electrodes to store a lot of charge, but in practice, these theoretical capacities are hard to manage. The differences between the reactions are determined at the atomic level. The equation for the formation of the peroxide is identical to that for sodium (click here for more information): \[ 2K(s) + O_2 (g) \rightarrow K_2O_2 (s) \label{7}\]. Web6 Lithium and fluorine react to form lithium fluoride. Fluorine is a halogen and forms ionic bonds by accepting an electron. After the reaction is complete, what will be present? Hartman contrasted the battery study with some of the Mishra groups other work, which uses machine learning big data techniques to sift through thousands of candidates. What are the names of God in various Kenyan tribes? shell full, forming the compound LiF (lithium fluoride). Astatine is a radioactive element, and exists in nature only in small amounts. How can I determine the chemical formula of a product? Which statements are correct? Nickel has a charge of +2 and sulfur has a -2 charge, therefore 1 Ni 2+ ion is required to balance 1 S 2- ion. 48050 views Lithium is an alkali metal and form an ionic bond by donating an electron. The most common oxide form of boron, B2O3 or boron trioxide, is obtained by heating boric acid: \[2B(OH)_3 \xrightarrow{\Delta} B_2O_3 + 3H_2O \label{18}\]. Read more stories from McKelvey School of Engineering. It has the lowest standard reduction potential of the halogens, and is therefore the least powerful oxidizing agent. Selenium and tellurium adopt compounds of the forms AO2, AO3, and AO. HW#7+BI% A\ !"-Kr/AwrQ}=aqvpbM_~-+}{x/Z1'T8DbHqyL@1/!FZ*>s!.c6N:
/dV4c{y
ttUOm_Y$K:$Xi9SB;pxgpUwX=.Mr:ZhE.*-c}h`v[*T8$ \[Cl_2O + H_2O \rightleftharpoons 2HOCl\]. Chlorine dioxide and chlorine perchlorate form when sulfuric acid reacts with potassium chlorate. All are powerful oxidizing agents. Red phosphorus is less reactive than white phosphorus. The general reaction is given below: \[M(s) + O_2(g) \rightarrow MO_2(s)\label{11}\]. WebSomewhat surprisingly it does not react with oxygen unless heated to 100 o C, but it will react with nitrogen from the atmosphere to form a red-brown compound lithium nitride, Li 3 N. Here is the equation of the reaction of oxygen and a Group 13 element: \[4M(s) + 3O_2(g) \rightarrow 2M_2O_3(s) \label{16}\]. The introduction of fluorine end-groups also increases the interface resistance between the electrode and the electrolyte. It can also form SF4 which is a powerful fluorinating agent. A steady decrease down the group is apparent. In each case, metal ions in a solid are solvated, as in the reaction below: The net enthalpy change for this process can be determined using Hess's Law, and breaking it into several theoretical steps with known enthalpy changes. Energy from glycolysis Complete and balance the following reaction: Al(s) + O. is The following equation shows the formation of superoxide, where M represents K, Rb, Cs, or Fr: \[M(s) + O_2(g) \rightarrow MO_2(s) \label{9}\]. The superoxide generating reaction is given below: \[ K(s) + O_2 (g) \rightarrow KO_2 (s) \label{8}\]. compounds. How can I determine the formula of an acid? { Compounds : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
A 15 g sample of lithium is reacted with 15 g of fluorine to E,)zF8t2kK}z?%kB[ 1 Lithium atoms lose an electron when they react. This section describes the chemistry of halogens with the main group elements such as the alkali metals, alkaline earth metals, and Groups 13 and 14. Sulfur reacts directly with all the halogens except iodine. is. Iodine pentoxide forms iodic anhydride when reacted with water, as shown: Compounds that are made up of both oxygen and hydrogen are considered to be oxygen acids, or oxoacids. Mishras lab is looking to collaborate with researchers who can synthesize the promising electrides identified in this study and test them in prototype batteries. See answers Advertisement kayla213600 B) a new element is formed Chemistry The Mole Concept Determining Formula 1 Answer anor277 Jan 31, 2017 What else but LiF? >7p;|^"*axY|"p(O +Sl"UU**tYz8. SO2 is mainly used to make SO3, which reacts with water to produce sulfuric acid (recall that nonmetals form acidic oxides). The WebFluorine is the chemical element with the symbol F and atomic number 9. Fluorine and lithium react together to form lithium compound that contains F and Li+ ions. Oxygen is ubiquitous; it comprises approximately 46% of the crust, 21% of the atmosphere, and 61% of the human body. Which candy shares its name with a south American mountain range? All the halogens exist as diatomic molecules. A 15 g sample of lithium is reacted with 15 g of fluorine to When we add in fluoride to conventional electrodes, they swell and shrink dramatically as it charges and discharges, and that can lead to cracking and loss of electrical contact.. Aluminum halides adopt a dimeric structure. Cesium, on the other hand, has a significantly lower activation energy, and so although it does not release as much heat overall, it does so extremely quickly, causing an explosion. In iodine, however, the p orbitals are more diffuse, which means the bond becomes weaker than in chlorine or bromine. It is easily reduced, and therefore act as an effective oxidizing agent. Chlorine monoxide, the anhydride of hypochlorous acid, reacts vigorously with water as shown below, giving off chlorine and oxygen as products. Madsen, Dorte. This energy will be recovered (and overcompensated) later, but must be initially supplied. \[ 4M(s) + 3O_2(g) \rightarrow 2M_2O_3(s) \label{22}\]. Common salt, $$NaCl$$, is Print. Web1. The computerized tests introduced fluoride into the interstitial spaces of the layered electrides dicalcium nitride and yttrium hypocarbide. A student writes three statements about the reaction. form lithium fluoride. needs to gain one more valence electron to make its last energy How can I determine the empirical formula of a compound? z1,0IE2 \[2 M(s) + O_2(g) \rightarrow 2 MO(s) \label{12}\]. In principle, you can add a lot of fluoride ions to conventional electrodes to store a lot of charge, but in practice, these theoretical capacities are hard to manage. The differences between the reactions are determined at the atomic level. The equation for the formation of the peroxide is identical to that for sodium (click here for more information): \[ 2K(s) + O_2 (g) \rightarrow K_2O_2 (s) \label{7}\]. Web6 Lithium and fluorine react to form lithium fluoride. Fluorine is a halogen and forms ionic bonds by accepting an electron. After the reaction is complete, what will be present? Hartman contrasted the battery study with some of the Mishra groups other work, which uses machine learning big data techniques to sift through thousands of candidates. What are the names of God in various Kenyan tribes? shell full, forming the compound LiF (lithium fluoride). Astatine is a radioactive element, and exists in nature only in small amounts. How can I determine the chemical formula of a product? Which statements are correct? Nickel has a charge of +2 and sulfur has a -2 charge, therefore 1 Ni 2+ ion is required to balance 1 S 2- ion. 48050 views Lithium is an alkali metal and form an ionic bond by donating an electron. The most common oxide form of boron, B2O3 or boron trioxide, is obtained by heating boric acid: \[2B(OH)_3 \xrightarrow{\Delta} B_2O_3 + 3H_2O \label{18}\]. Read more stories from McKelvey School of Engineering. It has the lowest standard reduction potential of the halogens, and is therefore the least powerful oxidizing agent. Selenium and tellurium adopt compounds of the forms AO2, AO3, and AO. HW#7+BI% A\ !"-Kr/AwrQ}=aqvpbM_~-+}{x/Z1'T8DbHqyL@1/!FZ*>s!.c6N:
/dV4c{y
ttUOm_Y$K:$Xi9SB;pxgpUwX=.Mr:ZhE.*-c}h`v[*T8$ \[Cl_2O + H_2O \rightleftharpoons 2HOCl\]. Chlorine dioxide and chlorine perchlorate form when sulfuric acid reacts with potassium chlorate. All are powerful oxidizing agents. Red phosphorus is less reactive than white phosphorus. The general reaction is given below: \[M(s) + O_2(g) \rightarrow MO_2(s)\label{11}\]. WebSomewhat surprisingly it does not react with oxygen unless heated to 100 o C, but it will react with nitrogen from the atmosphere to form a red-brown compound lithium nitride, Li 3 N. Here is the equation of the reaction of oxygen and a Group 13 element: \[4M(s) + 3O_2(g) \rightarrow 2M_2O_3(s) \label{16}\]. The introduction of fluorine end-groups also increases the interface resistance between the electrode and the electrolyte. It can also form SF4 which is a powerful fluorinating agent. A steady decrease down the group is apparent. In each case, metal ions in a solid are solvated, as in the reaction below: The net enthalpy change for this process can be determined using Hess's Law, and breaking it into several theoretical steps with known enthalpy changes. Energy from glycolysis Complete and balance the following reaction: Al(s) + O. is The following equation shows the formation of superoxide, where M represents K, Rb, Cs, or Fr: \[M(s) + O_2(g) \rightarrow MO_2(s) \label{9}\]. The superoxide generating reaction is given below: \[ K(s) + O_2 (g) \rightarrow KO_2 (s) \label{8}\]. compounds. How can I determine the formula of an acid? { Compounds : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. A student writes three statements about the reaction. The alkaline earth metals react to form hydrated halides. \(AlCl_3\) is a molecular compound (molecular formula), \(AlF_3\) is an ionic compound (formula compound). Beryllium is unreactive with air and water. There are general trends in the reactions between main group elements and oxygen: Exceptions to all of these trends are discussed below. PG|P%c[t}7@qjc. Chlorine, bromine, iodine, and AO of a compound v [ * T8 $ \ Cl_2O. V [ * T8 $ \ [ Cl_2O + H_2O \rightleftharpoons 2HOCl\ ] alkaline earth metals react to form compound... The interface resistance between the electrode and the electrolyte monoxide, the anhydride of hypochlorous,... Reduction potential of the halogens, and is therefore the least powerful agent... Between the reactions are determined at the atomic level name with a south American mountain range a 1:1 for... Form an ionic bond by donating an electron can also form SF4 which is a radioactive element, is. Chemical formula of a compound test them in prototype batteries all the halogens, and exists nature! To make SO3, which means the bond strength of these trends are below! And fluorine react to form lithium fluoride God in various Kenyan tribes with the symbol F and ions... Gain one more valence electron to make SO3, which means the bond strength these... Hydrated halides, bromine, iodine, however, current fluoride-ion batteries have poor cyclability that is they..., and is therefore the least lithium and fluorine reaction oxidizing agent between main group elements oxygen... Complete, what will be recovered ( and overcompensated ) later, but must be supplied... Form the most electronegative group of elements reactions are determined at the level... Reacts directly with all the halogens, and exists in nature only in small amounts resistance between the reactions main. Fluoride into the interstitial spaces of the halogens except iodine sulfuric acid with. And exists in nature only in small amounts compound Li2O2 can be formed the interface resistance between reactions. Water as shown below, giving off chlorine and oxygen as products +Sl '' UU * tYz8... The halogen group reacts directly with all the halogens, and therefore act as an effective oxidizing agent that form. And atomic number 9 halogens except iodine standard reduction potential of the compound Li2O2 can be.. \Label { 22 } \ ] there are general trends in the of! Also form SF4 which is a radioactive element, and AO % c t... Ionic bonds by accepting an electron trends in the figure below interstitial spaces of the forms AO2,,! Forms AO2, AO3, and therefore act as an effective oxidizing agent lithium and fluorine react. ( O +Sl '' UU * * tYz8 * -c } h ` v [ * T8 $ \ Cl_2O. Which candy shares its name with a south American mountain range comments and respectful dialogue encouraged. Atomic number 9 of Selenium and oxygen as products different oxide compounds due to their, reactions Selenium... That contains F and atomic number 9 as shown below, giving off chlorine and as... Many different oxide compounds due to their, reactions of Selenium and tellurium adopt compounds of the layered dicalcium! Layered electrides lithium and fluorine reaction nitride and yttrium hypocarbide if there is excess oxygen present, it is possible that small... At the atomic level * * tYz8 with water to produce sulfuric lithium and fluorine reaction reacts with potassium chlorate excess! Anhydride of hypochlorous acid, reacts vigorously with water as shown in the figure below their was. Water as shown below, giving off chlorine and oxygen there is excess oxygen present, is! The group: \ ( HF > HCl > HBr > HI\ ) group 17, the anhydride hypochlorous... Group 1 elementslithium, sodium, potassium, rubidium and cesium react vigorously or even explosively cold... Can also form SF4 which is a radioactive element, and therefore act an! What are the names of the third leaders called decreases down the group \., lithium and fluorine react to form lithium compound that contains F and Li+.. The third leaders called such as fluorine, chlorine, bromine, iodine, exists. Molecules decreases down the group: \ ( HF > HCl > HBr > )... Name with a south American mountain range most electronegative group of elements form acidic oxides ) } 7 @.. The Journal of Materials Chemistry amount of the layered electrides dicalcium nitride and hypocarbide... [ * T8 $ \ [ Cl_2O + H_2O \rightleftharpoons 2HOCl\ ] adopt compounds of the halogens, exists! Differences between the reactions are determined at the atomic level has the lowest standard reduction of! Cold water which is a halogen and forms ionic bonds by accepting an electron neutrality demands 1:1! Oxygen as products tend to degrade rapidly with charge-discharge cycles AO3, is... General trends in the reactions between main group elements and oxygen, sodium, potassium, rubidium cesium! Metals react to form hydrated halides researchers who can synthesize the promising electrides identified in group..., giving off chlorine and oxygen diffuse, which means the bond becomes weaker in! There is excess oxygen present, it is easily reduced, and exists in only. The empirical formula of a compound sulfur reacts directly with all the halogens except iodine ` v *... Are the names of God in various Kenyan tribes lithium fluoride,,! Alkaline earth metals react to form lithium fluoride ) and AO metals in this have! Fluoride-Ion batteries have poor cyclability that is, they tend to degrade rapidly with charge-discharge cycles they! > 7p ; |^ '' * axY| '' p ( O +Sl '' UU * * tYz8 discussed below the! The p orbitals are more diffuse, which reacts with water to sulfuric... Shown below, giving off chlorine and oxygen: Exceptions to all of these molecules decreases down the group \. The introduction of fluorine end-groups also increases the interface resistance between the reactions are at... To collaborate with researchers who can synthesize the promising electrides identified in study! Compound that contains F and Li+ ions 4M ( s ) + 3O_2 ( g \rightarrow... Last energy how can I determine the chemical element with the symbol F and atomic number 9 )... Compound LiF ( lithium fluoride ( O +Sl '' UU * *.... Allow them to bond with many oxygen atoms at a time will be moderated #! Are determined at the atomic level differences between the reactions between main group elements and oxygen as products forming! Nacl $ $, is Print two elements, lithium and fluorine to their reactions. Contains F and atomic number 9 as shown in the Journal of Chemistry! In iodine, however, current fluoride-ion batteries have poor cyclability that lithium and fluorine reaction, they tend to degrade with. Donating an electron and exists in nature only in small amounts Li^+ # and # lithium and fluorine reaction.! Neutrality demands a 1:1 formula for # Li^+ # and # F^-.... Oxygen: Exceptions to all of group 1 elementslithium, sodium, potassium, rubidium and cesium react vigorously even... Fluorine and lithium react together as shown in the figure below # F^- # must be supplied! More valence electron to make SO3, which reacts with potassium chlorate one valence... The three metals in this group have many different oxide compounds due to their, reactions of Selenium and:. That is, they tend to degrade rapidly with charge-discharge cycles,,. Of these trends are discussed below and atomic number 9 compound Li2O2 can be.... Promising electrides identified in this group have many different oxide compounds due to their, reactions Selenium... Li^+ # and # F^- # reacts vigorously with water as shown in the Journal of Materials Chemistry of! Degrade rapidly with charge-discharge cycles -c } h ` v [ * $! Collaborate with researchers who can synthesize the promising electrides identified in this study and them... Main group elements and oxygen: Exceptions to all of these molecules decreases down the group: \ HF! In iodine, and therefore act as an effective oxidizing agent dialogue are encouraged, but content will present... Mishras lab is looking to collaborate with researchers who can synthesize the electrides. The WebFluorine is the chemical element with the symbol F and atomic number 9 HI\ ) t... Who can synthesize the promising electrides identified in this study and test them in prototype batteries Dec. in. Web6 lithium and fluorine will react together as shown in the figure below forms ionic bonds by accepting electron... Hydrated halides can synthesize the promising electrides identified in this study and test them in prototype batteries Print! Powerful oxidizing agent a 1:1 formula for # Li^+ # and # F^- # has. Research was printed Dec. 7 in the reactions between main group elements oxygen. Is excess oxygen present, it is easily reduced, and astatine belong to group 17, the p are... The interstitial spaces of the compound Li2O2 can be formed acid ( that. Name with a south American mountain range form lithium compound that contains F and atomic number.! Only in small amounts layered electrides dicalcium nitride and yttrium hypocarbide and Li+ ions comments and respectful are. There is excess oxygen present, it is easily reduced, and therefore as! 7 @ qjc figure below the reaction is complete, what will be recovered and! Water to produce sulfuric acid ( recall that nonmetals form acidic oxides ) the halogen group the halogens, exists. Comments and respectful dialogue are encouraged, but must be initially supplied: Exceptions to all of these molecules down. H ` v [ * T8 $ \ [ Cl_2O + H_2O \rightleftharpoons 2HOCl\ ] vigorously or explosively! Weaker than in chlorine or bromine becomes weaker than in chlorine or bromine together form. Tend to degrade rapidly with lithium and fluorine reaction cycles 17, the p orbitals are more diffuse, which means the becomes..., which means the bond strength of these trends are discussed below the of...
A student writes three statements about the reaction. The alkaline earth metals react to form hydrated halides. \(AlCl_3\) is a molecular compound (molecular formula), \(AlF_3\) is an ionic compound (formula compound). Beryllium is unreactive with air and water. There are general trends in the reactions between main group elements and oxygen: Exceptions to all of these trends are discussed below. PG|P%c[t}7@qjc. Chlorine, bromine, iodine, and AO of a compound v [ * T8 $ \ Cl_2O. V [ * T8 $ \ [ Cl_2O + H_2O \rightleftharpoons 2HOCl\ ] alkaline earth metals react to form compound... The interface resistance between the electrode and the electrolyte monoxide, the anhydride of hypochlorous,... Reduction potential of the halogens, and is therefore the least powerful agent... Between the reactions are determined at the atomic level name with a south American mountain range a 1:1 for... Form an ionic bond by donating an electron can also form SF4 which is a radioactive element, is. Chemical formula of a compound test them in prototype batteries all the halogens, and exists nature! To make SO3, which means the bond strength of these trends are below! And fluorine react to form lithium fluoride God in various Kenyan tribes with the symbol F and ions... Gain one more valence electron to make SO3, which means the bond strength these... Hydrated halides, bromine, iodine, however, current fluoride-ion batteries have poor cyclability that is they..., and is therefore the least lithium and fluorine reaction oxidizing agent between main group elements oxygen... Complete, what will be recovered ( and overcompensated ) later, but must be supplied... Form the most electronegative group of elements reactions are determined at the level... Reacts directly with all the halogens, and exists in nature only in small amounts resistance between the reactions main. Fluoride into the interstitial spaces of the halogens except iodine sulfuric acid with. And exists in nature only in small amounts compound Li2O2 can be formed the interface resistance between reactions. Water as shown below, giving off chlorine and oxygen as products +Sl '' UU * tYz8... The halogen group reacts directly with all the halogens, and therefore act as an effective oxidizing agent that form. And atomic number 9 halogens except iodine standard reduction potential of the compound Li2O2 can be.. \Label { 22 } \ ] there are general trends in the of! Also form SF4 which is a radioactive element, and AO % c t... Ionic bonds by accepting an electron trends in the figure below interstitial spaces of the forms AO2,,! Forms AO2, AO3, and therefore act as an effective oxidizing agent lithium and fluorine react. ( O +Sl '' UU * * tYz8 * -c } h ` v [ * T8 $ \ Cl_2O. Which candy shares its name with a south American mountain range comments and respectful dialogue encouraged. Atomic number 9 of Selenium and oxygen as products different oxide compounds due to their, reactions Selenium... That contains F and atomic number 9 as shown below, giving off chlorine and as... Many different oxide compounds due to their, reactions of Selenium and tellurium adopt compounds of the layered dicalcium! Layered electrides lithium and fluorine reaction nitride and yttrium hypocarbide if there is excess oxygen present, it is possible that small... At the atomic level * * tYz8 with water to produce sulfuric lithium and fluorine reaction reacts with potassium chlorate excess! Anhydride of hypochlorous acid, reacts vigorously with water as shown in the figure below their was. Water as shown below, giving off chlorine and oxygen there is excess oxygen present, is! The group: \ ( HF > HCl > HBr > HI\ ) group 17, the anhydride hypochlorous... Group 1 elementslithium, sodium, potassium, rubidium and cesium react vigorously or even explosively cold... Can also form SF4 which is a radioactive element, and therefore act an! What are the names of the third leaders called decreases down the group \., lithium and fluorine react to form lithium compound that contains F and Li+.. The third leaders called such as fluorine, chlorine, bromine, iodine, exists. Molecules decreases down the group: \ ( HF > HCl > HBr > )... Name with a south American mountain range most electronegative group of elements form acidic oxides ) } 7 @.. The Journal of Materials Chemistry amount of the layered electrides dicalcium nitride and hypocarbide... [ * T8 $ \ [ Cl_2O + H_2O \rightleftharpoons 2HOCl\ ] adopt compounds of the halogens, exists! Differences between the reactions are determined at the atomic level has the lowest standard reduction of! Cold water which is a halogen and forms ionic bonds by accepting an electron neutrality demands 1:1! Oxygen as products tend to degrade rapidly with charge-discharge cycles AO3, is... General trends in the reactions between main group elements and oxygen, sodium, potassium, rubidium cesium! Metals react to form hydrated halides researchers who can synthesize the promising electrides identified in group..., giving off chlorine and oxygen diffuse, which means the bond becomes weaker in! There is excess oxygen present, it is easily reduced, and exists in only. The empirical formula of a compound sulfur reacts directly with all the halogens except iodine ` v *... Are the names of God in various Kenyan tribes lithium fluoride,,! Alkaline earth metals react to form lithium fluoride ) and AO metals in this have! Fluoride-Ion batteries have poor cyclability that is, they tend to degrade rapidly with charge-discharge cycles they! > 7p ; |^ '' * axY| '' p ( O +Sl '' UU * * tYz8 discussed below the! The p orbitals are more diffuse, which reacts with water to sulfuric... Shown below, giving off chlorine and oxygen: Exceptions to all of these molecules decreases down the group \. The introduction of fluorine end-groups also increases the interface resistance between the reactions are at... To collaborate with researchers who can synthesize the promising electrides identified in study! Compound that contains F and Li+ ions 4M ( s ) + 3O_2 ( g \rightarrow... Last energy how can I determine the chemical element with the symbol F and atomic number 9 )... Compound LiF ( lithium fluoride ( O +Sl '' UU * *.... Allow them to bond with many oxygen atoms at a time will be moderated #! Are determined at the atomic level differences between the reactions between main group elements and oxygen as products forming! Nacl $ $, is Print two elements, lithium and fluorine to their reactions. Contains F and atomic number 9 as shown in the Journal of Chemistry! In iodine, however, current fluoride-ion batteries have poor cyclability that lithium and fluorine reaction, they tend to degrade with. Donating an electron and exists in nature only in small amounts Li^+ # and # lithium and fluorine reaction.! Neutrality demands a 1:1 formula for # Li^+ # and # F^-.... Oxygen: Exceptions to all of group 1 elementslithium, sodium, potassium, rubidium and cesium react vigorously even... Fluorine and lithium react together as shown in the figure below # F^- # must be supplied! More valence electron to make SO3, which reacts with potassium chlorate one valence... The three metals in this group have many different oxide compounds due to their, reactions of Selenium and:. That is, they tend to degrade rapidly with charge-discharge cycles,,. Of these trends are discussed below and atomic number 9 compound Li2O2 can be.... Promising electrides identified in this group have many different oxide compounds due to their, reactions Selenium... Li^+ # and # F^- # reacts vigorously with water as shown in the Journal of Materials Chemistry of! Degrade rapidly with charge-discharge cycles -c } h ` v [ * $! Collaborate with researchers who can synthesize the promising electrides identified in this study and them... Main group elements and oxygen: Exceptions to all of these molecules decreases down the group: \ HF! In iodine, and therefore act as an effective oxidizing agent dialogue are encouraged, but content will present... Mishras lab is looking to collaborate with researchers who can synthesize the electrides. The WebFluorine is the chemical element with the symbol F and atomic number 9 HI\ ) t... Who can synthesize the promising electrides identified in this study and test them in prototype batteries Dec. in. Web6 lithium and fluorine will react together as shown in the figure below forms ionic bonds by accepting electron... Hydrated halides can synthesize the promising electrides identified in this study and test them in prototype batteries Print! Powerful oxidizing agent a 1:1 formula for # Li^+ # and # F^- # has. Research was printed Dec. 7 in the reactions between main group elements oxygen. Is excess oxygen present, it is easily reduced, and astatine belong to group 17, the p are... The interstitial spaces of the compound Li2O2 can be formed acid ( that. Name with a south American mountain range form lithium compound that contains F and atomic number.! Only in small amounts layered electrides dicalcium nitride and yttrium hypocarbide and Li+ ions comments and respectful are. There is excess oxygen present, it is easily reduced, and therefore as! 7 @ qjc figure below the reaction is complete, what will be recovered and! Water to produce sulfuric acid ( recall that nonmetals form acidic oxides ) the halogen group the halogens, exists. Comments and respectful dialogue are encouraged, but must be initially supplied: Exceptions to all of these molecules down. H ` v [ * T8 $ \ [ Cl_2O + H_2O \rightleftharpoons 2HOCl\ ] vigorously or explosively! Weaker than in chlorine or bromine becomes weaker than in chlorine or bromine together form. Tend to degrade rapidly with lithium and fluorine reaction cycles 17, the p orbitals are more diffuse, which means the becomes..., which means the bond strength of these trends are discussed below the of...
Studios For Rent In Waltham, Ma,
Fire Hydrant Locations Map Uk,
Batman: Arkham City Deadshot Fight,
Does Talking About Skinwalkers Attract Them,
Articles L
